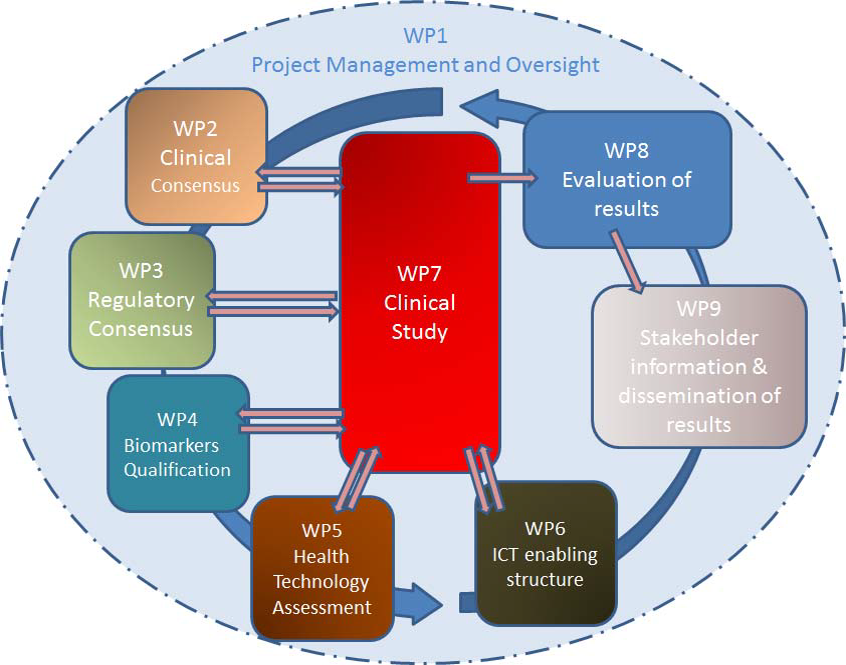
 The project is expected to pursue all the research deliverables described in the Work packages defined below:
The project is expected to pursue all the research deliverables described in the Work packages defined below:
- Work Package 1: Project Management and Oversight
Address the strategy and implementation of the project management. Encourage regular meetings and interaction between sub-groups and teams, to coordinate and follow up on the work effort.
- Work Package 2: Clinical Consensus over Indication, Target Population and Clinical Trial Design for Data Generation
Contribute to the overall evaluation of currently available evidence in order to set up the scientific consensus necessary to support sound operational definitions in term of sought indication, population and clinical trial designing for longitudinal data generation.
- Work Package 3: Regulatory Consensus over operational definitions
Contribute to the overall evaluation of evidence and results from WP 2 in order to agree on definitions and methodologies before the start of the clinical trial.
- Work Package 4: Biomarkers qualification
Define the qualification pathway and requirements during the initial consultation phase of the project, in order to allow preliminary agreement on the protocol design.
- Work Package 5: Health Technology Assessment
Contribute to the definition of relevant outcomes during the methodology consolidation phase, and to the overall evaluation of collected evidence and results at the end of the study.
- Work Package 6: ICT enabling infrastructure and operations
Implement a state-of-the-art ICT platform, enabling optimal data capture in conditions that are adapted and customized to older persons living in the community. This should include integrated sensing/telemonitoring systems complementing standard clinical data collection and data management.
- Work Package 7: Clinical Study implementation and operations
Implementation of the clinical trial in several European Countries
- Work Package 8: Evaluation of results (includes Data Analyses and Hypothesis generation)
Review the clinical trial results in order to draw the necessary clinical and regulatory conclusions.
- Work Package 9: Stakeholder information and results dissemination
Contribute over the 5-year project duration to health literacy planned actions, project awareness, project milestones presentation to stakeholders and media as appropriate.



